A Simple Flow Cytometry-Based Assay for CAR Detection Using Fluorokines™
CAR-T cell therapy is a form of cancer immunotherapy that has been remarkably successful for treating certain hematologic cancers. It is based on the principle that the body’s own T cells can be genetically modified to amplify the immune response and effectively eliminate cancer cells. CAR-T cell therapy relies on the ability to remove a patient’s or donor’s T cells and genetically engineer them to express a chimeric antigen receptor (CAR). CARs consist of an extracellular antibody-derived single chain variable fragment (scFv) that binds to a specific target antigen linked to one or more intracellular signaling sequences that promote immune cell activation following scFv binding. The patient’s T cells are transduced with the CAR using a retrovirus or lentivirus, and the resulting CAR-T cells are expanded ex vivo. Once a sufficient number of cells is obtained, the CAR-T cells are then infused back into the patient, where the CAR will bind to its target antigen expressed on the surface of the patient’s tumor cells, resulting in T cell activation and tumor cell destruction.

One of the current challenges in the field of CAR-T cell therapy is the lack of a simple assay to easily detect and quantify CAR expression following T cell transduction. This is essential for evaluating the transduction efficiency and determining the percentage of CAR+ T cells obtained. To address this need, Bio-Techne now offers R&D Systems™ Fluorokines for CAR detection. Fluorokines are recombinant proteins labeled with a fluorescent dye that allow target cells expressing the corresponding CAR to be directly stained and detected by flow cytometry. R&D Systems fluorescent-labeled proteins are rigorously tested for specificity, bioactivity, and lot-to-lot consistency to ensure maximum performance and data reproducibility. As the data in this application note shows, Fluorokines are also compatible with antibody panels, making it possible to more fully characterize CAR-T cells using multi-color flow cytometry.
R&D Systems Fluorokines Undergo Rigorous Testing to Ensure Specificity, Bioactivity, and Lot-to-Lot Consistency
The specificity of R&D Systems fluorescent-labeled proteins is confirmed by testing that each protein binds to beads conjugated to the corresponding monoclonal antibody. When possible, Fluorokines are also tested to ensure that they stain the appropriate CAR-T cells by flow cytometry. Additionally, minimal lot-to-lot variability is ensured both by maintaining consistent manufacturing conditions and by testing each new lot side-by-side with previous lots and with a master lot, to give researchers confidence that their results will be reproducible over time.
Specificity and Lot-to-Lot Consistency Testing of Recombinant Human CD19 Fc Chimera Atto 647N Protein

Analysis of the Specificity and Lot-to-Lot Consistency of Recombinant Human CD19 Fc Chimera Atto 647N Protein. CD4+CD8+ T cells were either (A) transduced with a hCD19-CAR or (B) not transduced, and then cultured for 11 days. Cells were stained with a PE-Cy7-CD4 and Recombinant Human CD19 Fc Chimera Atto 647N Protein (R&D Systems, Catalog # ATM9269) and detected by flow cytometry. (C) Streptavidin-coated beads conjugated to a Biotinylated Anti-Human CD19 Monoclonal Antibody (R&D Systems, Catalog # FAB4867B) were stained with the indicated concentrations of two independent lots of Recombinant Human CD19 Fc Chimera Atto 647N Protein (R&D Systems, Catalog # ATM9269). The blue and green bars on the graph represent data obtained from Recombinant Human CD19 Fc Chimera Atto 647N Protein from a different manufacturing run, demonstrating the lot-to-lot consistency of the protein.

Specificity, Bioactivity, and Lot-to-Lot Consistency Testing of Recombinant Human BCMA Fc Chimera Atto 647N Protein
Analysis of the Specificity, Bioactivity, and Lot-to-Lot Consistency of Recombinant Human BCMA/TNFRSF17 Fc Chimera Atto 647N Protein. Fluorescent beads conjugated to an anti-Human BCMA Monoclonal Antibody were stained with (A) Recombinant Human BCMA/TNFRSF17 Fc Chimera Atto 647N Protein (R&D Systems, Catalog # ATM193) or (B) unstained and detected by flow cytometry. (C) Recombinant Human APRIL/TNFSF13 (R&D Systems, Catalog # 5860-AP) was immobilized at 0.1 ug/mL, 100 ul/well, and the indicated concentrations of three independent lots of Recombinant Human BCMA Fc Chimera Atto 647N (R&D Systems, Catalog # ATM193; red, green, orange lines) or unlabeled Recombinant Human BCMA Fc Chimera (R&D Systems, Catalog # 193-BC; blue line) were added. The data demonstrates consistent bioactivity between the fluorescent-labeled and unlabeled proteins and lot-to-lot consistency in the bioactivity of the three different lots of the fluorescent-labeled protein.
Fluorokines Are Compatible with Antibody Panels for Multi-Color Flow Cytometry
Fluorokines are conjugated to Alexa Fluor® 488, Alexa Fluor® 647, Atto 488, or Atto 647N dyes, which offer intense fluorescence and excellent photostability. As shown in the data below, Fluorokines can be used in combination with antibody panels, making it possible to more fully characterize CAR-T cells by multi-color flow cytometry. Additionally, no secondary antibody is needed for Fluorokine detection, eliminating the possibility of background staining that may occur using an epitope-tagged target antigen and a fluorophore-labeled secondary antibody.
Characterization of CD4+ and CD8+ hCD19-CAR-T Cells by Multi-Color Flow Cytometry Using the Recombinant Human CD19 Fc Chimera Atto 647N Protein and a Panel of Fluorochrome-Conjugated Antibodies

Fluorokines Can be Used with Fluorochrome-conjugated Antibodies to Characterize CD19-CAR-T Cells by Multi-Color Flow Cytometry. Human PBMC CD4+CD8+ T cells transduced with a hCD19-CAR were stained with the following panel of monoclonal antibodies: Alexa Fluor® 700-conjugated Mouse Anti-Human CD14 (R&D Systems, Catalog # FAB3832N), PerCP-conjugated Mouse Anti-Human CD45 (R&D Systems, Catalog # FAB1430C), PE-conjugated Rabbit Anti-Human CD56/NCAM-1 (R&D Systems, Catalog # FAB24086P), Alexa Fluor® 405-conjugated Mouse Anti-Human CD8 (R&D Systems, Catalog # FAB1509V), PE-Cy7-CD4, and Alexa Fluor® 594-conjugated Mouse Anti-Human CD62L/L-Selectin (R&D Systems, Catalog # FAB9787T), along with the Fluorokine, Recombinant Human CD19 Fc Chimera Atto 647N Protein (R&D Systems, Catalog # ATM9269) and detected by flow cytometry. The results indicate that 43.5% of the CD4+ T cells and 15.6% of the CD8+ T cells were transduced with the hCD19-CAR. After staining with the Recombinant Human CD19 Fc Chimera Atto 647N Fluorokine, 36.7% of the CD4+hCD19-CAR+ T cells and 31.2% of the CD8+hCD19-CAR+ T cells were central memory T cells, indicated by positive staining for CD62L/L-Selectin. Cells were initially gated on singlets and live cells.
Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
Characterization of Naïve and Memory CD4+ and CD8+ hCD19-CAR-T Cells Following Staining with Recombinant Human CD19 Fc Chimera Alexa Fluor® 488 Protein
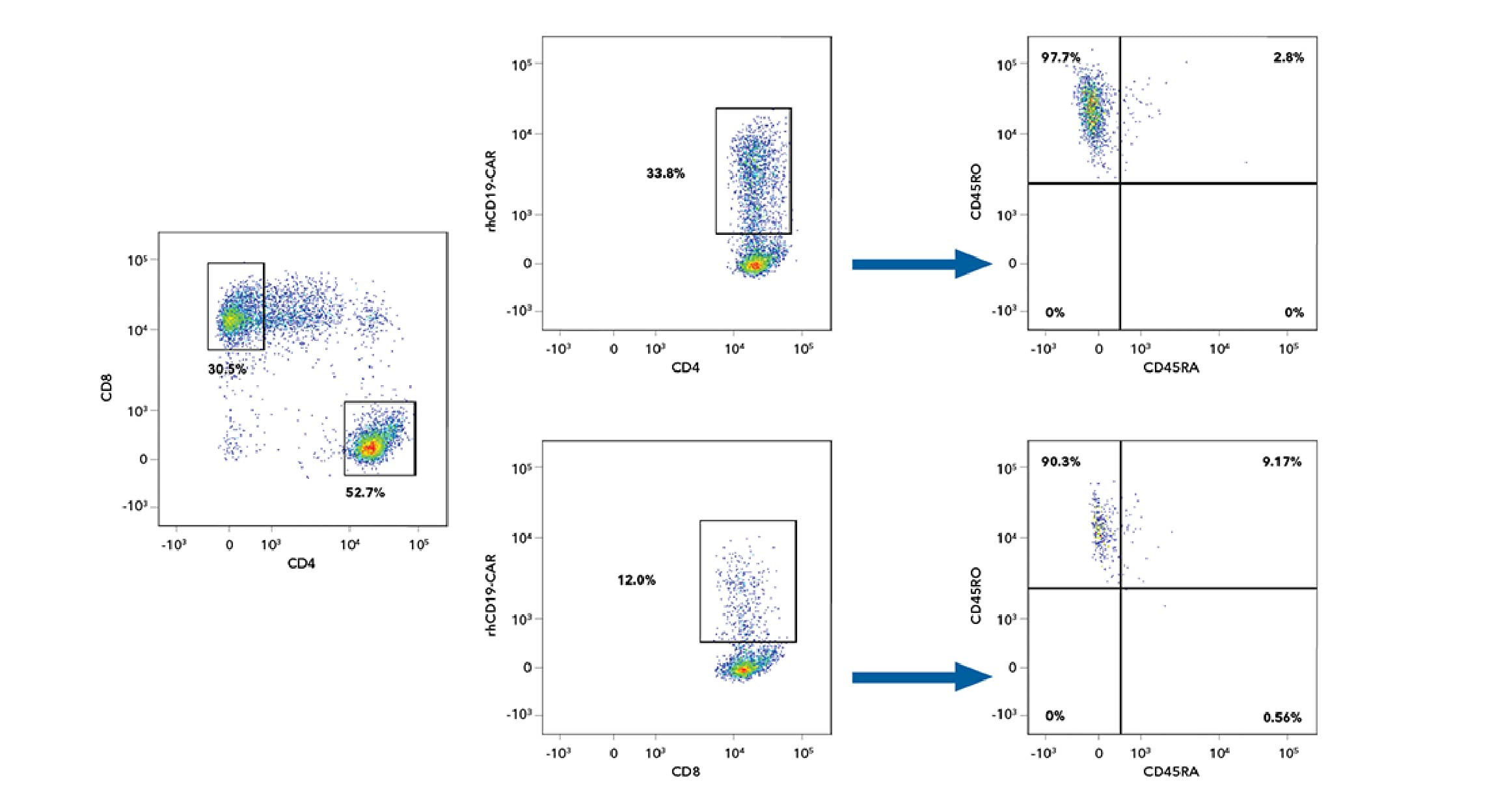
Identification of the Percentage of Naïve and Memory CD4+ and CD8+ CD19-CAR-T Cells After Staining with Alexa Fluor 488-labeled Recombinant Human CD19. Human PBMC CD4+CD8+ T cells transduced with a hCD19-CAR were stained with the following panel of monoclonal antibodies: PE-Cy7-CD4, Alexa Fluor® 405-conjugated Mouse Anti-Human CD8 (R&D Systems, Catalog # FAB1509V), APC-H7-CD45RA, and PE-conjugated Mouse Anti-Human CD45RO (R&D Systems, Catalog # FAB10642P), along with the Fluorokine, Recombinant Human CD19 Fc Chimera Alexa Fluor® 488 Protein (R&D Systems, Catalog # AFG9269) and detected by flow cytometry. The results indicate that 97.7% of the CD4+hCD19-CAR+ T-cells and 90.3% of the CD8+hCD19-CAR+ T cells were CD45RO+ after staining with the Recombinant Human CD19 Fc Chimera Alexa Fluor 488 Fluorokine, indicating that the majority of the cells were memory T cells. Cells were initially gated on singlets and live cells.
Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
Summary of the Benefits of Using Fluorokines for CAR Detection
- Fluorokines are a new line of R&D Systems fluorescent-labeled recombinant proteins that allow chimeric antigen receptors (CARs) to be directly stained and detected by flow cytometry.
- Fluorokines are rigorously tested for specificity and to ensure that they retain the same high level of activity as the corresponding unlabeled recombinant proteins.
- Each new protein lot is tested side-by-side with previous lots and with a master lot to ensure high lot-to-lot consistency.
- Fluorokines are compatible with antibody panels for multi-color flow cytometry, making it possible to more fully characterize CAR-T cells.
- No secondary antibody is needed for detecting Fluorokines, reducing processing time and eliminating the possibility of background staining that may occur by indirect detection using a secondary antibody.







