Simple Plex™ HIV p24 Assay
Precise and Reliable Lentivirus Titer
The HIV p24 antigen is a common Lentivirus (LVV) capsid protein used to quantify lentivirus titer in cell therapy applications. With the growing utilization of lentiviral vectors in in vivo and ex vivo gene delivery for developing treatments aimed at acquired and common genetic diseases, it is imperative that the production of clinical-grade LVV products adheres to rigorous standards in manufacturing and quality analysis. Detection of the HIV p24 antigen is commonly used for quantifying lentivirus titer in these applications.
The Simple Plex HIV p24 assay on the Ella platform offers a reliable and efficient method for detection of HIV p24 with an extended 4 log dynamic range to minimize sample dilutions. Learn more about the Ella platform here.
Why Use Ella for Lentivirus Titration?
Precision Results in Less Than 90 Minutes
Precision Results in Less Than 90 Minutes
Ella automated ELISAs precisely control all steps of the immunoassay, reducing errors and providing more consistent results. This, combined with built in standard curves, reduces the time to results allowing you to get more done.
Minimize Dilutions With Ultra-Wide Dynamic Range
Minimize Dilutions With Ultra-Wide Dynamic Range
Simple Plex HIV p24 assay offers a reliable and efficient method for detection of HIV p24 with an extended 4 log dynamic range to minimize sample dilutions.
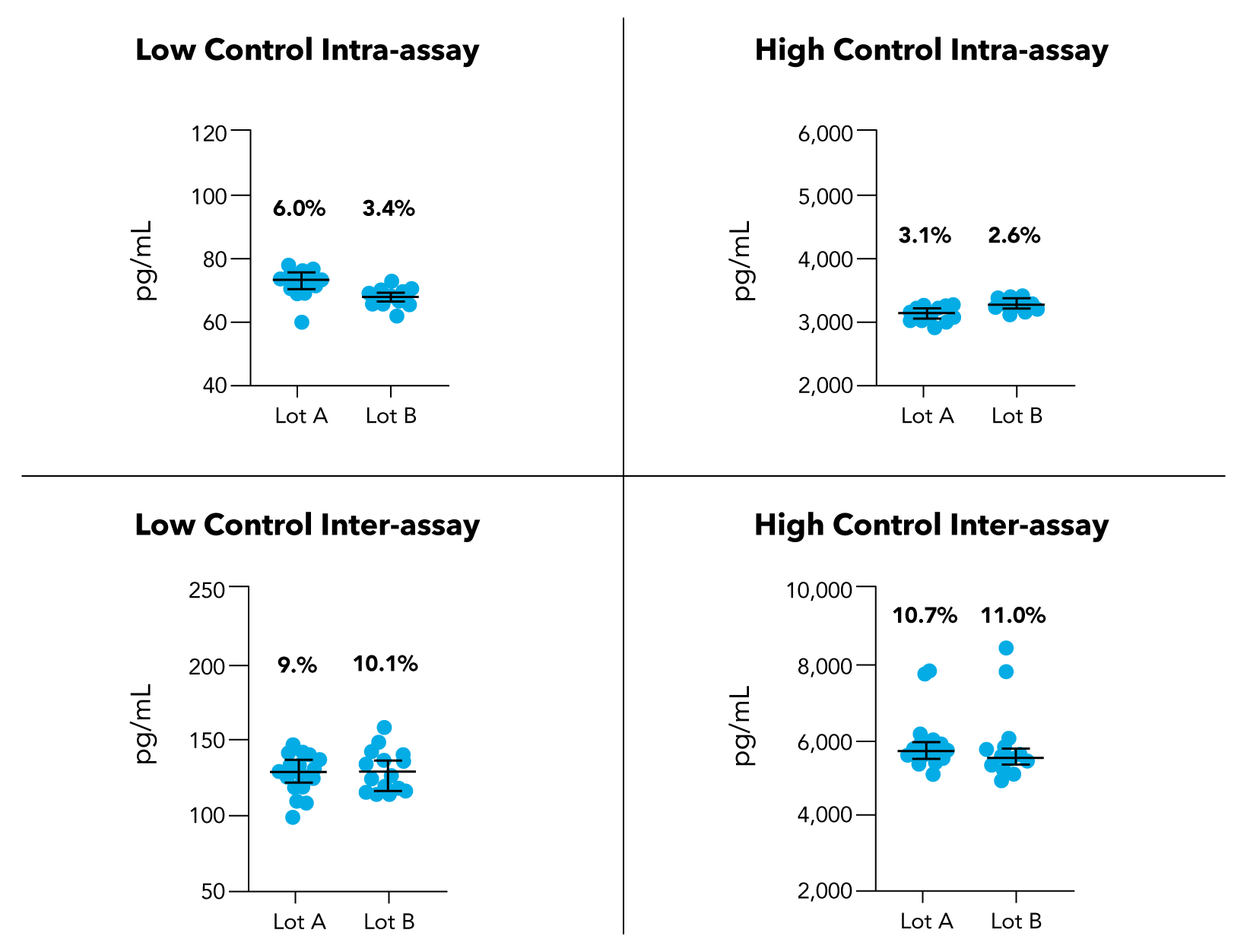
Simple Plex HIV p24 Reproducibility
Superior Precision. Reproducible Results.
In a study conducted by 7 operators using 11 Ella instruments, and with multiple preparations, the Simple Plex HIV-1 p24 assay has demonstrated high precision and reproducibility. The automated, microfluidic design of the Simple Plex assays running on Ella helps eliminate user-introduced variability by offering built-in standard curves and separate channels for each analyte, which also ensures no cross-reactivity. The results for the low control and high control intra and inter assays were reproducible with an overall coefficient of variation (CV) of between 2% and 11%.
Demonstrated Consistency
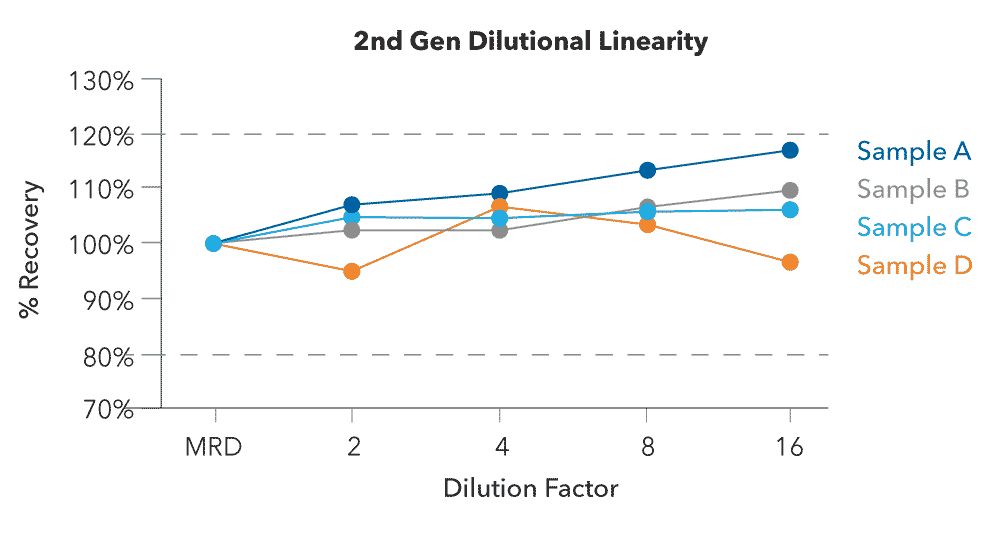
The Simple Plex HIV-1 p24 assay has demonstrated impressive linearity to showcase the accuracy within the expected assay range. The diluents are selected specific to each assay and are optimized to reduce matrix effects. The results for the five diluted points show that the concentration of the assay can be diluted and continue to provide reliable results. The recovery of each dilution point is well within the 80% and 120% range of expected values. The automated microfluidic cartridge is designed to allow operators to eliminate the lengthy incubations typical of traditional plate-based immunoassays while ensuring precise and consistent results across multiple users.
Additional Ella Resources

Meet Ella, Your Immunoassay Problem-Solver
With the Simple Plex HIV-1 p24 assay on Ella, you get measurements on an automated, robust, and reproducible immunoassay platform. Learn how Ella can bring a higher level of speed, precision, and efficiency to your lab, boosting your productivity.

Custom Simple Plex Assay Panels
Build your own multiplex biomarker immunoassay panel for Ella. Simple Plex assays offer consistent factory calibration, built-in standard curves, and precisely controlled automation, helping to ensure consistent results no matter the lab. Easily configure panels of up to 8 analytes with the matrix of your choice. Choose from more than 250 fully validated targets. The easy-to-use Panel Builder tool ensures assay compatibility.






