Stem cells are characterized as precursor cells that have the ability to self-renew and differentiate into multiple mature cell types. Many different stems cell types have been identified including embryonic stem cells, adult stem cells, hematopoietic stem cells, mesenchymal stem cells, and neural stem cells. These cells are being widely studied due to their potential applications in regenerative medicine and cellular therapies, as well as disease modeling and drug discovery.
Most stem cell studies rely on the ability to isolate and culture different stem cell types in vitro, which can be technically challenging. As a result, the first step in working with stem cells is typically identifying and optimizing appropriate culture conditions. Different stem cell types require specialized culture conditions to either expand the cells in vitro or promote their differentiation into specific cell lineages. Once appropriate culture conditions are identified, researchers have to confirm that these conditions are reproducible, so that they can standardize their protocols and minimize variability from one experiment to the next.
Cytokines and growth factors are some of the most important components of stem cell cultures. Different combinations of these factors are commonly used to promote either self-renewal or differentiation into specific cell lineages. As a result, the proteins added to stem cell cultures need to display high levels of activity, lot-to-lot consistency, and be free of contaminants to ensure that they efficiently drive the desired experimental outcome and contribute to the establishment of optimal, reproducible culture conditions. R&D Systems understands the importance of consistency and reproducibility in cell cultures. Our rigorous in-house testing and quality control specifications are designed to ensure that our proteins will provide the highest levels of performance, low endotoxin levels (<0.1 EU/ug), and minimal lot-to-lot variability. With over 30 years of experience purifying and manufacturing recombinant proteins, we offer stability of source and the expertise necessary to provide superior products that allow researchers to be confident in their stem cell culture conditions and the data that they generate.
Several Stem Cell Core Facility Directors Explain Why They Choose R&D Systems Cytokines
Directors at several different stem cell core facilities describe their experiences using R&D Systems cytokines and growth factors. The speakers on the video include Sunita D’Souza, Director of the Center for Modern Pediatric Diseases at St. Jude’s Medical Center, Deborah French, Director of the Human Pluripotent Stem Cell Core at the Children’s Hospital of Philadelphia, Barbara Corneo, Director of the Stem Cell Core Facility at Columbia University Medical School, and Wenli Yang, Director of the iPSC Core Facility at the University of Pennsylvania. Learn why they use R&D Systems cytokines for their research.
Pluripotent Stem Cells
Cytokines for ESC and iPSC Expansion - Products by Molecule
| Activin A * | BMP-4 * | FGF basic/FGF2 * | Nodal | TGF-beta 1 * | Wnt-3a * |
Cytokines for Ectoderm Lineage Differentiation - Products by Molecule
| BMP-4 * | EGF * | FGF basic/FGF-2 * | Noggin * |
Cytokines for Mesoderm Lineage Differentiation - Products by Molecule
| Activin A * | BMP-2 * | BMP-4 * | Dkk-1 |
| FGF basic/FGF2 * | FGF-3 | FGF-10 | FGF-17 |
| Flt-3 Ligand * | IL-3 * | IL-6 * | IL-11 |
| Noggin * | PDGF-BB * | SCF/c-kit Ligand * | TGF-beta 1 * |
| Thrombopoietin/TPO * | VEGF* | Wnt-5a |
Cytokines for Endoderm Lineage Differentiation - Products by Molecule
| Activin A * | BMP-4 * | EGF * | FGF basic/FGF-2 * |
| FGF-4 | FGF-7/KGF * | FGF-10 | FGF-18 |
| HGF * | IGF-I/IGF-1 * | Noggin * | PDGF-BB * |
| Sonic Hedgehog/Shh * | VEGF* | Wnt-3a * |
*GMP-grade Proteins are available for these molecules.
New ExCellerate iPSC Expansion Medium
Supports robust expansion and maintenance of pluripotent stem cell culture for enhanced consistency and reproducibility.
- Animal component-free
- No growth factor supplementation required
- Stable cell integrity over long term culture
Assessment of the Bioactivity of R&D Systems Recombinant Human FGF2 and Wnt-3a

R&D Systems Recombinant Human FGF basic/FGF2 (146 aa) and Recombinant Human Wnt-3a Display Higher Activity than the Same Proteins Provided by Leading Competitors.
(A) The bioactivity of R&D Systems Recombinant Human FGF basic/FGF2 (146 aa) (Catalog # 233-FB; orange line) or recombinant human FGF basic/FGF2 (146 aa) from a leading competitor (green line) was assessed by measuring the ability of the proteins to stimulate proliferation of the NR6R-3T3 mouse fibroblast cell line. The bioactivity of R&D Systems Recombinant Human FGF basic/FGF2 (146 aa) was approximately 3-fold greater than the top competitor’s FGF basic (146 aa). (B) The bioactivity of R&D Systems Recombinant Human Wnt-3a (Catalog # 5036-WN; orange line) or recombinant human Wnt-3a from another company (green line) was assessed by measuring the ability of the proteins to induce alkaline phosphatase production in the MC3T3-E1 mouse preosteoblast cell line. The ED50 for this effect for R&D Systems Recombinant Human Wnt-3a was 1.7-fold better, with more than twice the maximum response compared to the competitor's Wnt-3a.
Differentiation of hESCs into Endoderm Induced by Recombinant Human Activin A

Differentiation of Human Embryonic Stem Cells in Media Supplemented with R&D Systems Recombinant Human Activin A.
BG01V human embryonic stem cells were (A) cultured in media without any protein added or (B) differentiated into endoderm using media supplemented with Recombinant Human Activin A (R&D Systems, Catalog # 338-AC). Differentiation into endoderm was confirmed by positive staining for Claudin-6 (red) and Sox17 (green) using the Mouse Anti-Human Claudin-6 Monoclonal Antibody (R&D Systems, Catalog # MAB3656) and the Goat Anti-Human Sox17 Polyclonal Antibody (R&D Systems, Catalog # AF1924), respectively. BG01V human embryonic stem cells are licensed from ViaCyte, Inc.
Neural Stem Cells
Cytokines for Neural Stem Cells - Products by Molecule
| BDNF | BMP-2 * | BMP-4 * | CNTF | EGF * | FGF basic/FGF2 * |
| FGF-4 | FGF-8a | FGF-8b | FGF-10 | GDNF * | IGF-I/IGF-1 * |
| beta-NGF | Noggin * | NT-4 | PDGF-BB * | Sonic Hedgehog/Shh * | VEGF* |
*GMP-grade Proteins are available for these molecules.
Analysis of the Bioactivity and Consistency of R&D Systems Recombinant Human Noggin

R&D Systems Recombinant Human Noggin Displays Minimal Lot-to-Lot Variability and Higher Activity than Leading Competitors’ Noggin Proteins.
BG01V human embryonic stem cells were cultured in Mouse Embryonic Fibroblast Conditioned Media supplemented with FGF basic (5 ng/mL). Stem cells were driven into early cells of the neuroectoderm using a 3-day incubation in Recombinant Human Noggin (25 ug/mL) from either R&D Systems (Lot 1, Lot 2; Catalog # 6057-NG) or from two different competitors (Competitor 1, Competitor 2). Control cells were incubated in media without Noggin (No Noggin). The cells were stained for the early ectoderm marker, Otx2, and the neuroectoderm marker, SOX1. (A) Representative images of SOX1 (green), Otx2 (red), and DAPI (blue) staining in embryonic stem cells differentiated with Noggin from R&D Systems or Noggin from Competitor 2. (B) SOX1+ clusters were quantified under each of the indicated culture conditions. Cells treated with R&D Systems Noggin showed an increase in SOX1+ cells compared to both untreated and competitor-treated cells. R&D Systems Noggin showed consistent differentiation across the lots tested. BG01V human embryonic stem cells were licensed from ViaCyte, Inc. (C) The bioactivity of R&D Systems Recombinant Human Noggin (Catalog # 6057-NG; orange line) or recombinant human noggin from a top competitor (green line) was determined by assessing the ability of the proteins to inhibit alkaline phosphatase production induced by 50 ng/mLRecombinant Human BMP-4 (R&D Systems, Catalog # 314-BP) in the ATDC5 mouse chondrogenic cell line. In the presence of 50 ng/mL Recombinant Human BMP-4, the ED50 for this effect for R&D Systems Recombinant Human Noggin was approximately 30-fold greater than the top competitor’s Noggin protein.
Protein Characterization Using SEC-MALS Analysis

A. Recombinant Human Noggin Protein SEC-MALS. Recombinant human Noggin (Catalog # 6057-NG) has a molecular weight (MW) of 58.9 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 12.6-13.2 min |
| MW-Predicted (Monomer) | 23.0 kDa |
| MW-MALS | 58.9 kDa |
| Polydispersity | 1.002 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
B. Recombinant Human GDNF Protein SEC-MALS. Recombinant human GDNF (Catalog # 212-GD) has a molecular weight (MW) of 27.5 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 18.8-19.4 min |
| MW-Predicted (Monomer) | 11.6 kDa |
| MW-MALS | 27.5 kDa |
| Polydispersity | 1.002 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
C. Recombinant Mouse Sonic Hedgehog/Shh (C25II), N-Terminus SEC-MALS Recombinant Mouse Sonic Hedgehog/Shh (C25II), N-Terminus (Catalog # 464-SH) has a molecular weight (MW) of 20.7 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 20.5-21.3 min |
| MW-Predicted (Monomer) | 19.8 kDa |
| MW-MALS | 20.7 kDa |
| Polydispersity | 1 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |

Recombinant Human IL‑10 Protein SEC-MALS. Recombinant human IL-10 (Catalog # 1064-ILB) has a molecular weight (MW) of 36.7 kDa as analyzed by SEC-MALS, suggesting that this protein is a homodimer. MW may differ from predicted MW due to post-translational modifications (PTMs) present (i.e. Glycosylation).
| SEC-MALS Data | Result |
| Retention Time | 17.9-18.5 min |
| MW-Predicted (Monomer) | 19.0 kDa |
| MW-MALS | 36.7 kDa |
| Polydispersity | 1.002 |
| System Suitability: BSA Monomer 66.4 ± 3.32 kDa | Pass |
Mesenchymal Stem Cells
Cytokines for Mesenchymal Stem Cells - Products by Molecule
| BMP-2 * | BMP-4 * | BMP-6 | EGF * | FGF basic/FGF2 * | FGF-4 |
| GDF-5/BMP-14 | HB-EGF | IGF-I/IGF-1 * | LR3 IGF-I/IGF-1 * | IL-6 * | PDGF-BB * |
| Sonic Hedgehog/Shh * | TGF-beta 1 * | TGF-beta 2 | TGF-beta 3 | Wnt-5b | Wnt-10b |
*GMP-grade Proteins are available for these molecules.
Analysis of the Bioactivities of R&D Systems Recombinant Human Shh Proteins
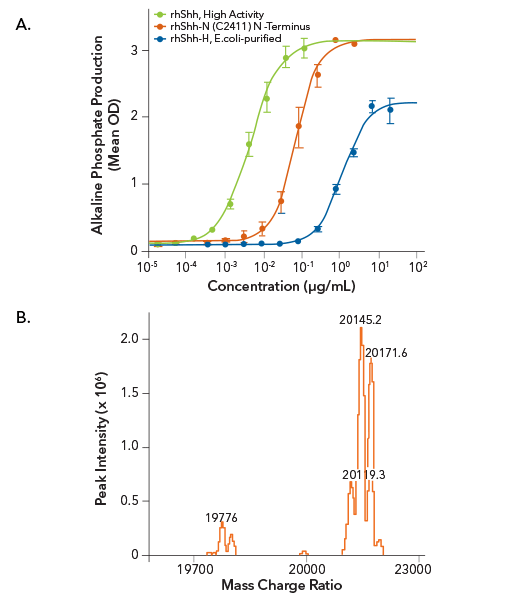
R&D Systems Recombinant Human Sonic Hedgehog/Shh Purified from HEK293 Cells and Containing the Correct Post-Translational Modifications Displays Higher Activity than E. coli-purified Recombinant Human Shh Proteins.
(A) The bioactivity of R&D Systems High Activity Recombinant Human Shh (Catalog # 8908-SH; green line), purified from HEK293 cells and containing the correct post-translational modifications (cholesterol and fatty acids), was compared to the bioactivity of E. coli-purified Recombinant Human Shh (C24II) N-terminus (R&D Systems, Catalog # 1845-SH; red line), and E.coli-purified Recombinant Human Shh, N-terminus (R&D Systems, Catalog # 1314-SH; blue line) by measuring the ability of the three proteins to induce alkaline phosphatase production by mesenchymal stem cells. The HEK293-purified High Activity Recombinant Human Shh protein was found to be over 14-fold more active than the E.coli-purified Recombinant Human Shh (C24II) N-Terminus protein, and over 250-fold more active than E. coli-purified Recombinant Human Shh-N protein. (B) LC/ESI-MS analysis of High Activity Recombinant Human Shh (R&D Systems, Catalog # 8908-SH) shows major peaks at 20119.3, 20145.2, and 20171.6 Da, suggesting that Recombinant Human Shh molecules are dual-modified with cholesterol at the C-terminus, and fatty acids (lauric acid, myristic acid, and palmitic acid) at the N-terminus. The minor peaks at 19776 Da corresponds to Recombinant Human Shh with only the fatty acid modification.
Hematopoietic Stem Cells
Cytokines for Hematopoietic Stem Cells - Products by Molecule
| BMP-4 * | Erythropoietin | Flt-3 Ligand * | G-CSF |
| GM-CSF * | IFN-gamma * | IL-2 * | IL-3 * |
| IL-4 * | IL-6 * | IL-7 * | IL-10 |
| IL-11 | IL-15 * | IL-21 * | M-CSF * |
| SCF/c-kit Ligand * | Thrombopoietin * | VEGF* | Wnt-3a * |
*GMP-grade Proteins are available for these molecules.
Assessment of the Bioactivity of R&D Systems Recombinant Human Thrombopoietin

R&D Systems Recombinant Human Thrombopoietin/Tpo Displays Higher Activity than Leading Competitors’ Thrombopoietin Proteins.
The bioactivity of R&D Systems Recombinant Human Thrombopoietin (Catalog # 288-TPE; orange line) or recombinant human Thrombopoietin/Tpo from two different competitors (green and purple lines) was assessed by measuring the ability of the proteins to stimulate proliferation of the MO7e human megakaryocytic leukemic cell line. The ED50 for this effect for R&D Systems Recombinant Human Thrombopoietin is 0.05-0.5 ng/mL, which is over 2-fold more active than the two competitors’ proteins.
Consistency Testing of R&D Systems GMP-grade Recombinant Human Flt-3 L and SCF

R&D Systems GMP-grade Recombinant Human Flt-3 Ligand and GMP-grade Recombinant Human SCF Display High Lot-to-Lot Consistency.
A) Three independent lots of GMP-grade Recombinant Human Flt-3 Ligand (R&D Systems, Catalog # 308E-GMP) were tested for their ability to stimulate proliferation of the BaF3 mouse pro-B cell line transfected with mouse Flt-3. The ED50 for this effect is 0.2-1 ng/mL. (B) Three independent lots of GMP-grade Recombinant Human SCF (R&D Systems, Catalog # BT-SCF-GMP) were tested for their ability to stimulate proliferation of the TF-1 human erythroleukemic cell line. The ED50 for this effect is 1-8 ng/mL. Each trace shown on the graphs represents data obtained from GMP-grade Recombinant Human Flt-3 Ligand or GMP-grade Recombinant Human SCF from a different manufacturing run to show the lot-to-lot consistency of the proteins.
Additional Stem Cell-Related Products
Products for Stem Cell Research
Products for Stem Cell Research
Minimize variability in your stem cell experiments and ensure high performance by using our stem cell culture reagents. From isolation and culture to verification, reprogramming, differentiation, and characterization, we have products that you need for your stem cell research.
Cell Culture Reagents
Cell Culture Reagents
Browse our complete collection of products for cell culture. We offer a comprehensive range of reagents to promote robust cell growth, including media and supplements, FBS, basement membrane extracts, and custom cell culture services.
Organoid and 3-D Cell Culture Products
Organoid and 3-D Cell Culture Products
Optimize your organoid culture conditions by using our complete range of products designed to support robust, reproducible organoid expansion. We provide basement membrane extracts, growth factors, small molecules, media, and media supplements, along with antibodies and RNAscope™ in situ hybridization assays for identifying lineage-specific markers.
Products for Regenerative Medicine
Products for Regenerative Medicine
Expand the frontiers in regenerative medicine with the power and flexibility of stem cells. We support regenerative medicine manufacturing with a vast array of products and services to help you advance and manufacture treatments for degenerative diseases, tissue injury, and genetic defects.
GMP Capabilities
GMP Capabilities
GMP ancillary and raw materials must provide robust performance in cell manufacturing workflows. With our focus on quality, innovation, supply chain continuity, and traceability, Bio-Techne GMP reagents are the reliable solution for your cell culture expansion and differentiation protocols.
Custom Solutions for Cell and Gene Therapy
Custom Solutions for Cell and Gene Therapy
Customization of raw materials enables cell therapy manufacturers to be confident in the security of their supply, reduces risks associated with manufacturing processes, and improves scalability. From GMP-grade proteins and antibodies to cell processing and custom chemistry services, we can develop the exact tools that you need for manufacturing your cell or gene therapy.