TR-FRET and FP Assay Reagents
TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer) and FP (Fluorescence Polarization) assays are widely used in biology and drug discovery to study target engagement. Bio-Techne offers a range of fluorescent probes suitable for the development of TR-FRET and FP assays.
| Product Name | Catalog # | Action |
|---|---|---|
| BDY FL Thalidomide | 7633 | High-affinity fluorescent cereblon probe for use in TR-FRET assays |
| BDY FL VH032 | 7483 | High-affinity VHL fluorescent probe for TR-FRET and FP assays |
| CELT-133 | 7952 | Selective hα1A adrenergic receptor fluorescent antagonist |
| CELT-327 | 7954 | Potent and selective hA2B/A3 adenosine receptor fluorescent antagonist |
| CELT-426 | 7955 | Potent and partially selective hD2 dopamine receptor fluorescent antagonist |
| CoraFluor™ 1, amine reactive | 7920 |
Terbium cryptate FRET donor for TR-FRET assay development; amine reactive for conjugation; |
| CoraFluor™ 2, amine reactive | 7950 |
Terbium cryptate FRET donor for TR-FRET assay development; amine reactive for conjugation; |
| FAM-DEALA-Hyp-YIPD | 7287 | Fluorescent HIF-1α peptide; can be used to assess VHL binding in FP assays |
| FAM-DEALAHypYIPMDDDFQLRSF | 7452 | Fluorescent HIF-1α peptide; can be used to assess VHL binding in FP assays |
| FITC-labelled Keap1-Nrf2 probe | 7627 | Fluorescent Keap1-Nrf2 peptide for use in TR-FRET and FP assays |
| Fluorescein-NAD+ | 6574 | Fluorescent NAD+; substrate for ADP-ribosylation for use in PARP assays; wavelength compatible with use as an acceptor dye in TR-FRET assays |
| JQ1-FITC | 7722 | Fluorescent BET bromodomain probe; suitable for use in TR-FRET |
| LDV FITC | 4577 | Fluorescent α4β1 integrin ligand; wavelength compatible with use as an acceptor dye in TR-FRET assays |
| PARPi-FL | 6461 | Fluorescent potent PARP inhibitor; wavelength compatible with use as an acceptor dye in TR-FRET assays |
| Thalidomide-Cyanine 5 | 7288 | High affinity cereblon fluorescent probe for use in TR-FRET |
| Tocrifluor T1117 | 2540 | Fluorescent cannabinoid CB1 receptor ligand; wavelength compatible with use as an acceptor dye in TR-FRET assays |
CoraFluor™ 1, amine reactive (Catalog # 7920) and CoraFluor™ 2, amine reactive (Catalog # 7950) are terbium-based probes that have been developed for use as TR-FRET donors. They emit wavelengths compatible with commonly used fluorescent acceptor dyes such as BODIPY® (or BDY) and Janelia Fluor® dyes, FITC (Catalog # 5440), TMR and Cyanine 5 (Catalog # 5436). CoraFluor fluorescence is brighter and more stable in biological media than existing TR-FRET donors, leading to enhanced sensitivity and improved data generation. CoraFluor™ 1 exhibits excitation upon exposure to a 337 nm UV laser, whereas CoraFluor™ 2 displays a red-shifted excitation wavelength, enhancing excitation efficiency at 365 nm and 405 nm. This attribute of CoraFluor™ 2 widens the range of analytical instruments suitable for these assays (FIGURE 1).

CoraFluor reagents can be conjugated directly to your antibody or protein of interest. We also offer custom conjugation of CoraFluor to an antibody of your choice.
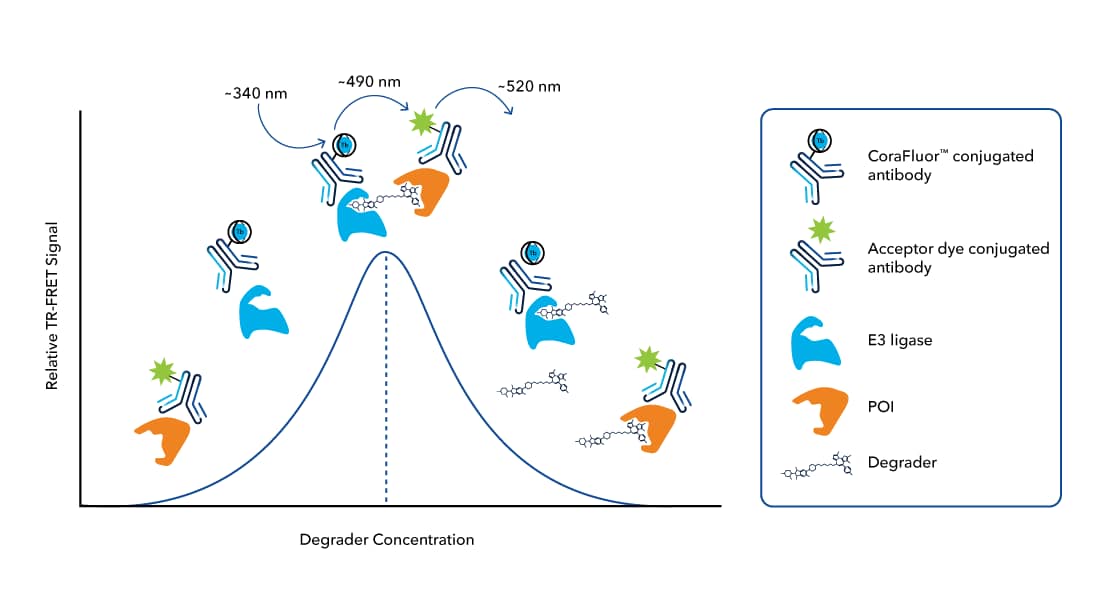
TR-FRET assays are useful in the study of ternary complex formation in the development of small molecule Protein Degraders, such as PROTAC® molecules. They provide a quantitative measure of the interactions between the Degrader, the target protein, and the ubiquitin E3 ligase (FIGURE 2). Characterizing the binding affinity of the Degrader to the target protein, the recruitment of ubiquitin E3 ligase to the target protein, and the formation of a co-operative ternary complex necessary for ubiquitination and subsequent degradation, using TR-FRET assays are critical for effective targeted protein degradation research, as this allows for optimization of Degrader molecules to improve their potency and selectivity.
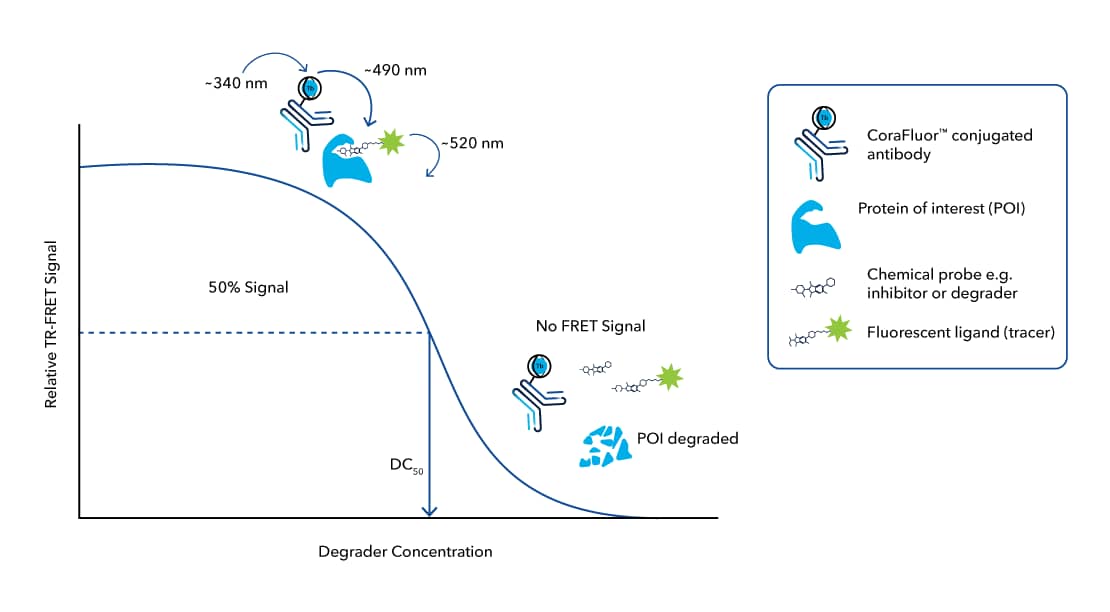
TR-FRET assays provide precise quantification of target protein levels in cellular lysates following application of a Degrader, which is essential for targeted protein degradation research. In addition, TR-FRET assays are appliable to a high-throughput format on a fluorescence microplate reader. This technique offers invaluable insights into the time- and dose-dependent kinetics of degradation for a panel of candidate Degraders. Furthermore, it enables calculation of important parameters, including the DC50 (the concentration at which target protein degradation is half-maximal) and Dmax (the maximal achievable degradation level) (FIGURE 3).
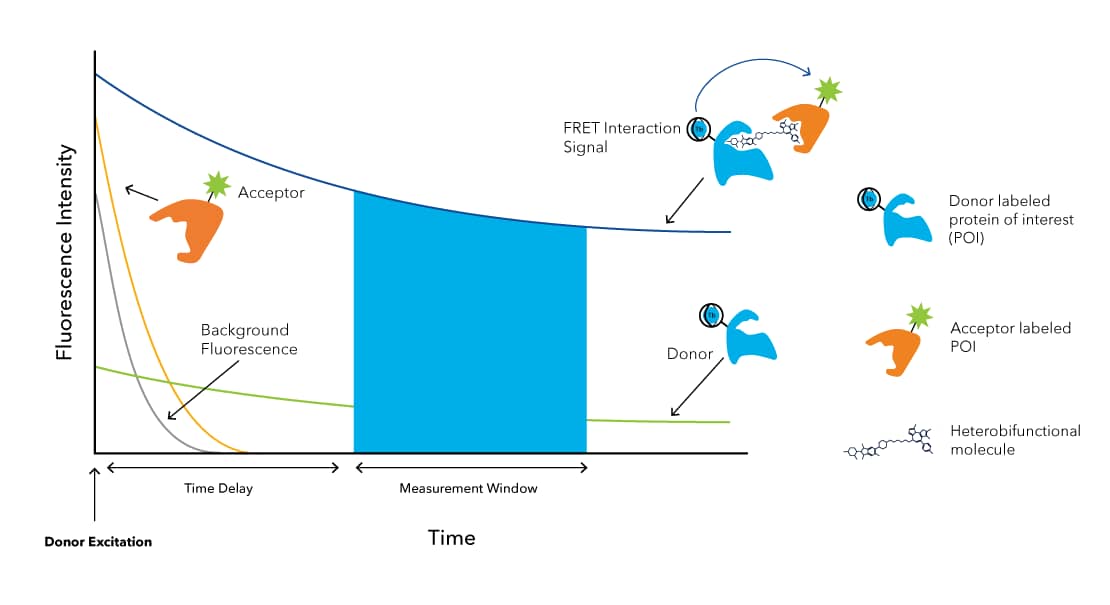
TR-FRET is a highly sensitive assay technology used in the field of drug discovery and biological research to study protein-protein interactions, enzyme kinetics, and receptor-ligand binding. This technology works by measuring the energy transfer between two fluorescent molecules: a donor fluorophore, such as CoraFluor, and an acceptor fluorophore. The approach involves excitation of the donor molecule, typically with a 340 nm light source; if the donor molecule and acceptor molecule are in proximity (5 – 10 nm), energy is transferred from the donor to the acceptor through a phenomenon called Förster resonance energy transfer (FRET) (FIGURE 4). Lanthanides, such as terbium or europium, are generally uses as donors in TR-FRET as they have a large Stokes shift and millisecond long lifetimes. This enables time-delayed detection and eliminates background noise derived from scattered excitation light and autofluorescence from media components and proteins.
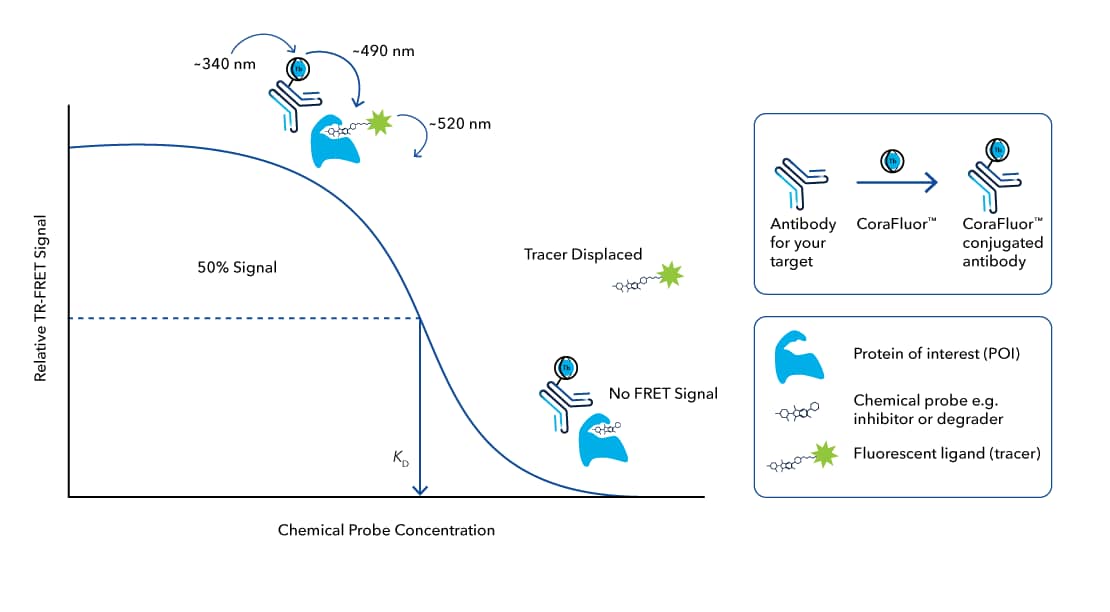
TR-FRET has been extensively used to identify novel drug targets, screen compound libraries, and optimize small molecules leads in various therapeutic areas, including oncology, cardiovascular disease, infectious disease, and neurodegenerative disorders. TR-FRET assays are particularly useful in measuring the binding affinity of small molecules, such as inhibitors and Degraders, to protein targets in a multi-well plate format, allowing for efficient high-throughput screening (HTS) of target engagement (FIGURE 5).
FP assays involve the excitation of a fluorescent molecule with polarized light and detection of emission using parallel and perpendicular polarizing filters. The technique is based on the observation that the effect of polarized light applied to a fluorescent probe is dependent on multiple factors, such as molecular conformation, orientation, and size. FP assays measure the change in orientation of a molecule in time, between absorption and emission events, and indicate the level of binding of the fluorescent molecule.
Like TR-FRET, fluorescence polarization (FP) assays are used in biology to study molecular interactions, such as protein-protein, protein-DNA, and protein-small molecule binding, as well as for monitoring the progress of enzyme reactions. The approach provides a rapid, quantitative, and versatile analysis of molecular interactions, and can be used to measure pharmacological parameters for small molecules, such as affinity constants (Ki, Kd) and IC50 values. FP assays are therefore frequently used in HTS for drug discovery.

Targeted Protein Degradation Research Product Guide
Our Targeted Protein Degradation Research Product Guide highlights the tools and services available from Bio-Techne to support your Targeted Protein Degradation research including, Active Degraders, Degrader Building Blocks, Custom Degrader Services, Proteins and Assays for protein degradation.
Webinar: 4th Bio-Techne Targeted Protein Degradation Symposium – Hear from leading scientists about the latest insights into protein degradation research
Learn more about our Targeted Protein Degradation products and services
BODIPY® is a registered trademark of Molecular Probes.
CoraFluor™ is a trademark of Bio-Techne Corp.
PROTAC® is a registered trademark of Arvinas Operations, Inc., and is used under license.





